 |
 |

In the spring, immediately after ice-out in temperate climates, the water column is cold and nearly isothermal with depth. The intense sunlight of spring is absorbed in the water column, which also heats up as the average daily temperature of the air increases. In the absence of wind, a temperature profile with depth might be expected to resemble Figure 2 (see the Light section), decreasing exponentially with depth. However, density, another physical characteristic of water, plays an important role in modifying this pattern.
Water differs from most other compounds because it is less dense as a solid than as a liquid. Consequently ice floats, while water at temperatures just above freezing sinks. As most compounds change from a liquid to a solid, the molecules become more tightly packed and consequently the compound is denser as a solid than as a liquid. Water, in contrast, is most dense at 4°C and becomes less dense at both higher and lower temperatures. The density/temperature relationship of fresh water is shown in Figure 3. Because of this density-temperature relationship, many lakes in temperate climates tend to stratify, that is, they separate into distinct layers.
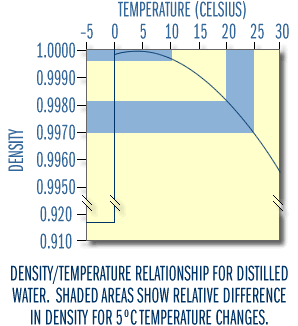
Figure 3
Spring
In lakes of the upper Midwest and at higher elevations, the water near a lake’s bottom will usually be at 4°C just before the lake's ice cover melts in the spring. Water above that layer will be cooler, approaching 0°C just under the ice. As the weather warms, the ice melts. The surface water heats up and therefore it decreases in density. When the temperature (density) of the surface water equals the bottom water, very little wind energy is needed to mix the lake completely. This is called turnover. After this spring turnover, the surface water continues to absorb heat and warms. As the temperature rises, the water becomes lighter than the water below. For a while winds may still mix the lake from bottom to top, but eventually the upper water becomes too warm and too buoyant to mix completely with the denser deeper water. As Figure 3 suggests, the relatively large differences in density at higher temperatures are very effective at preventing mixing. It simply takes too much energy to mix the water any deeper.
It is useful to visualize a more extreme example of density stratification. Imagine a bottle of salad dressing containing vegetable oil and vinegar. The oil is lighter (more buoyant) than the vinegar which is mostly water. When you shake it up you are supplying the energy to overcome the buoyant force, so the two fluids can be uniformly mixed together. However, if allowed to stand undisturbed, the more buoyant (less dense) oil will float to the top and a two-layer system will develop.
In some cases, such as happened at Ice Lake in April, 1998 and 1999, the surface water may warm up rapidly immediately after ice-out, causing the lake to stratify thermally without completely mixing. This prevents atmospheric oxygen from reaching the bottom waters. As a consequence, the entire water column never reaches 100% oxygen saturation. This can be observed for Ice Lake by comparing temperature and oxygen profiles from March 5, 1998 (still frozen), April 18, 1998 (the lake was completely ice-free on April 11, 1998), and April 30, 1998.
Summer
As summer progresses, the temperature (and density) differences between upper and lower water layers become more distinct. Deep lakes generally become physically stratified into three identifiable layers, known as the epilimnion, metalimnion, and hypolimnion (Figure 4). The epilimnion is the upper, warm layer, and is typically well mixed. Below the epilimnion is the metalimnion or thermocline region, a layer of water in which the temperature declines rapidly with depth. The hypolimnion is the bottom layer of colder water, isolated from the epilimnion by the metalimnion. The density change at the metalimnion acts as a physical barrier that prevents mixing of the upper and lower layers for several months during the summer.
The depth of mixing depends in part on the exposure of the lake to wind (its fetch), but is most closely related to the lake’s size. Smaller to moderately-sized lakes (50 to 1000 acres) reasonably may be expected to stratify and be well mixed to a depth of 3–7 meters in north temperate climates. Larger lakes may be well mixed to a depth of 10–15 meters in summer (e.g., Western Lake Superior near Duluth, MN).
Note that although "thermocline" is a term often used synonymously with metalimnion, it is actually the plane or surface of maximum rate of decrease of temperature with respect to depth. Thus, the thermocline is the point of maximum temperature change within the metalimnion.
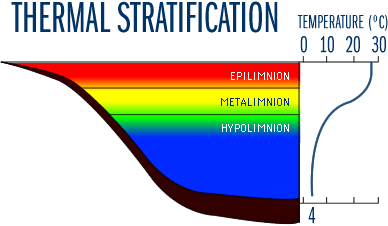
Figure 4
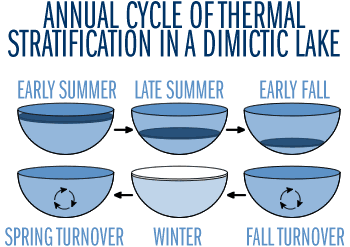
Figure 5
Autumn
As the weather cools during autumn, the epilimnion cools too, reducing the density difference between it and the hypolimnion (Figure 5). As time passes, winds mix the lake to greater depths, and the thermocline gradually deepens. When surface and bottom waters approach the same temperature and density, autumn winds can mix the entire lake; the lake is said to "turn over." As the atmosphere cools, the surface water continues to cool until it freezes. A less distinct density stratification than that seen in summer develops under the ice during winter. Most of the water column is isothermal at a temperature of 4°C, which is denser than the colder, lighter water just below the ice. In this case the stratification is much less stable, because the density difference between 0°C and 4°C water is quite small. However, the water column is isolated from wind-induced turbulence by its cap of ice. Therefore, the layering persists throughout the winter. You will need to download the Quicktime Plug-in from Apple to view these movies.
- Movie 1 - Here's what happens when warmer water (green) enters the surface of a lake in winter. The second addition shows that the warm water is buoyant (less dense) than the cold water and therefore rises.
- Movie 2 - Here's what happens when colder water enters a summer-stratified lake.
- Movie 3 - Same movie 2 without the dyed green epilimnion.
- Movie 4 - See what happens to the epilimnion (mixed layer) and thermocline during a storm. Did the lake mix?
- Movie 5 - Same as movie 4, but with increased turbulence. See what starts to happen when the class 5 tornado hits.
- Movie 6 - Shows how stream sediment entering a lake or reservoir deposits its load. Why does some material stay in the upper layer and some crash to the bottom?
- Movie 7 - An estuary is a 2-layer system with freshwater overlying salt water. Here we see how freshwater behaves when added to each layer.
- Movie 8 - Same as movie 7, but here we introduce water that is saltier than the upper freshwater layer. Example: Hurricanes can "throw" huge amounts of saltwater into coastal lakes. What happens to this water and what might its impact be?
Overview
This pattern (spring turnover — summer stratification — fall turnover — winter stratification) is typical for temperate lakes. Lakes with this pattern of two mixing periods are referred to as dimictic. Many shallow lakes, however, do not stratify in the summer, or stratify for short periods only, throughout the summer. Lakes that stratify and destratify numerous times within a summer are known as polymictic lakes. Both polymictic and dimictic lakes are common in Minnesota.
Since installing the RUSS unit in Ice Lake we have made an interesting observation. Spring turnover is incomplete. There was not enough mixing in spring, 1998 or 1999 to completely re-aerate the entire water column to 100% saturation. On the other hand, Lake Independence, a lake of comparable depth (15-18 meters) but much larger in size (more fetch) and less sheltered from the wind, mixed completely. We suspect that most aquatic scientists would not have expected to see Ice Lake’s bottom water, nearly saturated with oxygen in fall, 1998, to be anoxic by mid-winter and then persist in this state until the following fall. Once stratified thermally in summer, even the barrage of severe thunderstorms that occurred near Ice Lake in summer, 1999, lacked the energy to dramatically decrease the thermocline or increase the oxygen content of the hypolimnion. Heat and Oxygen budget section of Ice Lake.
It was cold and windy enough during fall, 1998 for Ice Lake to mix thoroughly, bringing oxygen to the bottom waters (to about 100% saturation). This is likely typical for Ice Lake during most autumns, although it is possible for a cold, calm period to allow the lake surface to freeze before the water column has been fully exposed to the atmosphere and re-charged with oxygen.
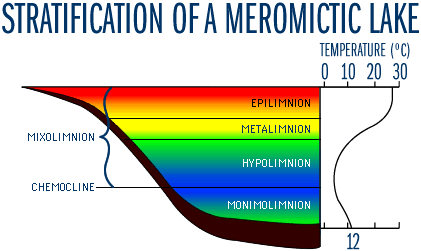
Figure 6
The West Upper Lake Station of Lake Minnetonka, Lake Independence and thousands of other Upper Midwestern lakes that are relatively deep (>10 meters) and reasonably large (>100-200 acres or 40-80 ha) are probably dimictic, leading to complete re-oxygenation of the water column for at least some period of time. Ice Lake, though small (41 acres or 16.6ha), is sheltered and deep (16 m) for its size. Lakes that have formed in former open pit mines in Northeastern Minnesota are unusually deep for their size. Lakes with these characteristics probably only mix completely once a year in the fall for a brief period before freezing. Some of the deeper mine pit lakes (>75 meters deep) probably never mix completely to the bottom, although data are sparse.
Much less common are lakes that circulate incompletely resulting in a layer of bottom water that remains stagnant. To distinguish them from the holomictic (mixing from top to bottom) lakes, these partially mixing lakes are referred to as meromictic. They mix partially, in the sense that they may have extensive mixing periods which go quite deeply into the hypolimnion, but they do not turn over completely, and a layer of bottom water remains stagnant and anoxic for years at a time. The non-mixing bottom layer is known as the monimolimnion and is separated from the mixolimnion (the zone that mixes completely at least once a year) by the chemocline (Figure 6). The stagnant, and typically anaerobic, monimolimnion has a high concentration of dissolved solids compared to the mixolimnion. In general, meromictic lakes have large relative depths. These lakes are typically small and sheltered from the wind by the morphology of their basin. In this case, the density differences caused by temperature are smaller than density differences due to the high dissolved solids (salts) concentration of the monimolimnion. Large lakes that rarely freeze over are also typically monomictic, mixing throughout the fall, winter and spring and stratifying in the summer.
To visualize this effect, try dissolving several tablespoons of table salt (NaCl) in hot water. Add a few drops of food coloring and then fill a mayonnaise jar half-full. Now, very gently add cool tap water with a small measuring cup to fill the glass. Set up a second jar half full with clear, cool water and then add the colored hot water to fill the glass - but don't add the salt. Compare the stability of the density stratification in the two systems by gently shaking or stirring the water columns.
|
MIXING
REGIME
|
LAKES |
MAX |
AREA |
|
DIMICTIC (2mixes/yr) |
Lake Minnetonka (Minneapolis, MN) |
34
|
14,004
acres (5,670 ha)
|
| Grindstone Lake (Sandstone, MN) |
46
|
500
acres (200 ha)
|
|
| Lake Independence (Minneapolis, MN) |
18
|
850
acres (344 ha)
|
|
| Pike Lake (Duluth, MN) |
18
|
500 acres (200 ha)
|
|
|
MONOMICTIC (1mix/yr
– mixed |
Lake Erie |
70
|
6.4 x 106 acres (2.6 x 106 ha) |
| Lake Huron |
228
|
14.8 x 106 acres (6.0 x 106 ha) | |
| Ice Lake* (Grand Rapids, MN) |
16
|
41
acres (16.6 ha)
|
|
| Lake Michigan |
281
|
14.4 x 106 acres (5.8 x 106 ha) | |
| Lake Ontario |
244
|
4.9 x 106 acres (2.0 x 106 ha) | |
| Lake Superior |
300
|
20.3
x 106 acres (8.2 x106 ha)
|
|
| Lake Tahoe (CA/NV) |
499
|
123,253
acres (49,900 ha)
|
|
| Lake Mead (NV – largest US reservoir) |
180
|
163,320
acres (66,096 ha)
|
|
|
POLYMICTIC (many mixes/yr) |
Shallow lakes & ponds |
<
4
|
wide
range
|
| Mille Lacs Lake, MN |
13
|
132,510 acres (53,648 ha) |
|
| St. Louis River and Duluth-Superior Harbor |
1-8
|
11,993
acres (4,856 ha)
|
|
|
MEROMICTIC (never
totally |
Miners Pit Lake (Ely, MN) |
48
|
138
acres (56 ha)
|
| Pennington Pit Lake, (Crosby, MN) |
79
|
57
acres (23 ha)
|
|
| Brownie Lake (Minneapolis, MN) |
15
|
18
acres (7.3 ha)
|
|
| Deming Lake (Itasca State Park, MN) |
14
|
12.3 acres (5.0 ha) |
|
| Big Soda Lake (Fallon, NV) |
60
|
400
acres (160 ha)
|
|
|
|||
For additional information, Learn about ARCHIMEDES's principle at EXPLORATORIUM and a shockwave demonstration of density and water displacement.